NEWTON, MA –May 16, 2022 – Acer Therapeutics Inc. (Nasdaq: ACER), a pharmaceutical company focused on the acquisition, development and commercialization of therapies for serious, rare and life-threatening diseases with significant unmet medical needs, today reported financial results for the first quarter ended March 31, 2022 and provided an update on the Company’s recent corporate developments.
“Progress in Q1 2022 was marked by a transformative debt financing in March, with continued advancement toward potential commercial launch of ACER-001 for UCDs and further development of our other pipeline programs,” said Chris Schelling, CEO and Founder of Acer. “Our advances to date have positioned Acer for potential achievement of a number of important planned milestones through the rest of 2022, including an FDA decision on our ACER-001 New Drug Application in June, the planned initiation of our EDSIVO™ pivotal Phase 3 trial in vascular Ehlers-Danlos Syndrome under our SPA agreement with FDA by end of Q2 2022, and ACER-801 Phase 2a trial results in Vasomotor Symptoms in H2 2022.”
Q1 2022 and Recent Highlights
- ACER-001 (sodium phenylbutyrate)
- Announced in April 2022 the presentation of data evaluating the bioavailability, bioequivalence and taste attributes of ACER-001 (sodium phenylbutyrate) compared to sodium phenylbutyrate (BUPHENYL®) powder during poster sessions at the Society for Inherited Metabolic Disorders (SIMD) Annual Meeting
- Announced in May 2022 the presentation of data evaluating the bioavailability, bioequivalence and taste attributes of ACER-001 (sodium phenylbutyrate) compared to sodium phenylbutyrate (BUPHENYL®) powder during poster sessions at the Genetic Metabolic Dieticians International (GMDI) Conference. Publications from SIMD and GMDI are available at: https://www.acertx.com/publications-and-presentations/
- Launched SeeUCDifferently, a national U.S. disease awareness campaign intended to provide education and information about urea cycle disorders (UCDs), including www.SeeUCDifferently.com, a new online resource that provides general UCDs diagnosis and disease education information
- ACER-801 (osanetant)
- Announced in March 2022 enrollment of the first patient in a Phase 2a (NCT05325775) randomized, double-blind, placebo-controlled, dose-ranging trial evaluating the pharmacokinetics (PK), safety, and efficacy of ACER-801 at different doses, compared to placebo, for the treatment of moderate to severe Vasomotor Symptoms (VMS) in post-menopausal women. Results from this trial could provide proof of concept data in post-menopausal women and could inform ACER-801 dosing and a development path forward in patients with induced Vasomotor Symptoms (iVMS)
- EDSIVO™ (celiprolol)
- In January 2022, the U.S. Food and Drug Administration (FDA) cleared the celiprolol IND for the treatment of patients with COL3A1-positive vascular Ehlers-Danlos Syndrome (vEDS)
- In April 2022, announced FDA granted celiprolol Breakthrough Therapy designation (BTD) in the U.S. for the treatment of patients with COL3A1-positive vEDS
- In May 2022, announced agreement with FDA under a Special Protocol Assessment (SPA) for pivotal Phase 3 clinical trial of celiprolol for the treatment of patients with COL3A1-positive vEDS. More information on the DiSCOVER (Decentralized Study of Celiprolol on vEDS-related Event Reduction) trial is available at https://discoverceliprolol.com/
- Corporate
- In February 2022, announced the appointment of Adrian Quartel, M.D., FFPM, as Chief Medical Officer. Dr. Quartel is an industry veteran with over 20 years of drug development experience tasked with overseeing Acer’s clinical development, medical affairs, regulatory affairs and other scientific and medical functions. Dr. Quartel joins Acer from Adamas Pharmaceuticals where he served as Chief Medical Officer overseeing research and development, as well as medical affairs and regulatory functions. Prior to Adamas, Dr. Quartel held senior medical leadership positions at BioMarin Pharmaceutical Inc., Astellas, Chiltern, and ICON Clinical Research
- In March 2022, announced convertible note and loan financing facilities for up to $48.5 million with affiliates of Marathon Asset Management L.P. (Marathon) and SWK Holdings Corporation, subject to certain conditions. Proceeds from these financings will be used to advance pipeline and for general corporate purposes. Further information with respect to the debt financing agreements with Marathon and SWK is contained in a Current Report on Form 8-K filed with the Securities and Exchange Commission on March 7, 2022
- Ended Q1 2022 with $20.8 million in cash and cash equivalents, which Acer believes will be sufficient to fund its currently anticipated operating and capital requirements into Q3 2022
Anticipated Milestones
- ACER-001 (sodium phenylbutyrate)
- June 5, 2022: FDA has assigned a PDUFA target action date of June 5, 2022, following its acceptance for filing of the NDA for ACER-001 for the treatment of patients with UCDs
- End of Q2 2022: Acer plans to submit an Investigational New Drug (IND) application by the end of Q2 2022 for a clinical study evaluating ACER-001 in Maple Syrup Urine Disease (MSUD)
- ACER-801 (osanetant)
- H2 2022: Results from the ongoing ACER-801 Phase 2a clinical trial in women with moderate to severe VMS are anticipated in H2 2022
- EDSIVO™ (celiprolol)
- End of Q2 2022: Acer intends to initiate by end of Q2 2022 the pivotal Phase 3, randomized, double-blind, placebo-controlled, decentralized clinical trial for celiprolol for patients with COL3A1-positive vEDS under its SPA agreement with FDA. The duration of the DiSCOVER clinical trial is currently estimated to be approximately 3.5 years to completion, once fully enrolled, and will require additional capital beyond Q3 2022
Q1 2022 Financial Results
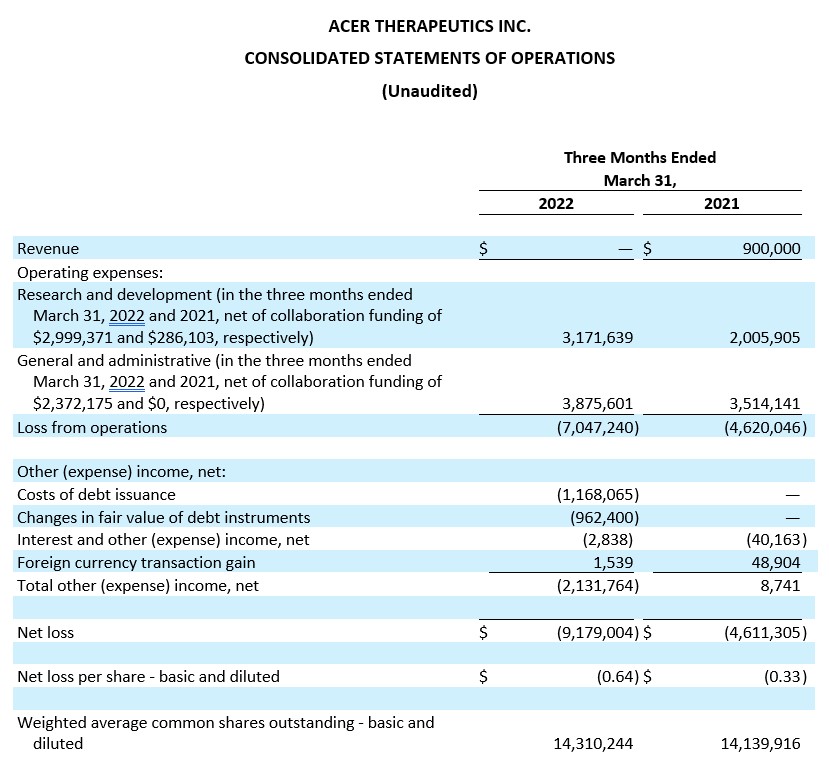
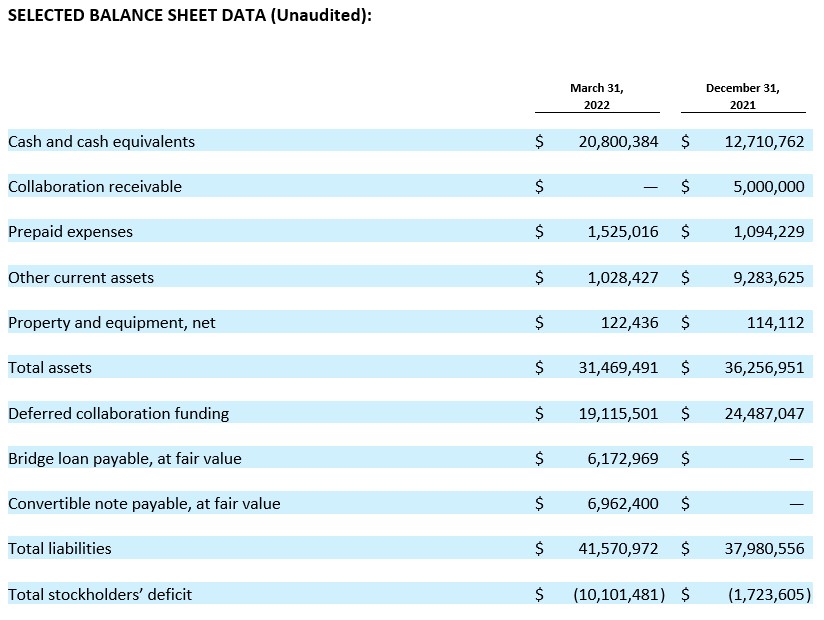
Cash position. Cash and cash equivalents were $20.8 million as of March 31, 2022, compared to $12.7 million as of December 31, 2021. Acer believes its cash and cash equivalents available as of March 31, 2022 will be sufficient to fund its currently anticipated operating and capital requirements into Q3 2022.
Research and Development Expenses. Research and development expenses were $3.2 million, net of collaboration funding of $3.0 million, for the three months ended March 31, 2022, compared to $2.0 million, net of collaboration funding of $0.3 million, for the three months ended March 31, 2021. This increase of $1.2 million was primarily due to increases in contract manufacturing expenses, employee-related expenses including a one-time bonus accrual, and clinical and medical affairs expenses, partially offset by the recognition of $3.0 million of the collaboration funding from the Collaboration Agreement with Relief. Research and development expenses for the three months ended March 31, 2022 were comprised of $3.1 million related to ACER-001, offset by $3.0 million of collaboration funding; $1.3 million related to ACER-801; $1.2 million related to EDSIVO™; $0.5 million related to other development activities.
General and Administrative Expenses. General and administrative expenses were $3.9 million, net of collaboration funding of $2.4 million for the three months ended March 31, 2022, compared to $3.5 million for the three months ended March 31, 2021. This increase of $0.4 million was primarily due to increases in precommercial expenses, audit and consulting fees, and employee-related expenses including a one-time bonus accrual, partially offset by the recognition of $2.4 million of the collaboration funding from the Collaboration Agreement with Relief.
Net Loss. Net loss for the three months ended March 31, 2022 was $9.2 million, or $0.64 net loss per share (basic and diluted), compared to a net loss of $4.6 million, or $0.33 net loss per share (basic and diluted), for the three months ended March 31, 2021.
For additional information, please see Acer’s Quarterly Report on Form 10-Q filed today with the SEC.
About Acer Therapeutics Inc.
Acer is a pharmaceutical company focused on the acquisition, development and commercialization of therapies for serious rare and life-threatening diseases with significant unmet medical needs. Acer’s pipeline includes four investigational programs: ACER-001 (sodium phenylbutyrate) for treatment of various inborn errors of metabolism, including urea cycle disorders (UCDs) and Maple Syrup Urine Disease (MSUD); ACER-801 (osanetant) for treatment of induced Vasomotor Symptoms (iVMS); EDSIVO™ (celiprolol) for treatment of vascular Ehlers-Danlos syndrome (vEDS) in patients with a confirmed type III collagen (COL3A1) mutation; and ACER-2820 (emetine), a host-directed therapy against a variety of viruses, including cytomegalovirus, zika, dengue, ebola and COVID-19. For more information, visit www.www.acertx.com.
Acer Forward-Looking Statements
This press release contains “forward-looking statements” that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in this press release regarding strategy, future operations, timelines for clinical study enrollment or regulatory actions, or otherwise, future financial position, future revenues, projected expenses, regulatory submissions, actions or approvals, cash position, liquidity, prospects, plans and objectives of management are forward-looking statements. Examples of such statements include, but are not limited to, statements relating to the potential for our investigational product candidates to safely and effectively treat diseases and to be approved for marketing; our ability to close upon and obtain the proceeds of any identified financing arrangements as well as to satisfy the ongoing conditions and requirements for maintaining the financing facilities and avoiding default or an accelerated payment requirement; the commercial or market opportunity of any of our product candidates in any target indication and any territory; our ability, in addition to the currently identified financings, to secure the additional capital necessary to fund our various product candidate development programs; the adequacy of our capital to support our future operations and our ability to successfully fund, initiate and complete clinical trials and regulatory submissions for ACER-001, ACER-801, EDSIVO™ or our other product candidates; the ability to protect our intellectual property rights; our strategy and business focus; and the development, expected timeline and commercial potential of any of our product candidates. Our pipeline products are under investigation and their safety and efficacy have not been established and there is no guarantee that any of our investigational products in development will receive health authority approval or become commercially available for the uses being investigated. We may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in the forward-looking statements and you should not place undue reliance on these forward-looking statements. Such statements are based on management’s current expectations and involve risks and uncertainties. Actual results and performance could differ materially from those projected in the forward-looking statements as a result of many factors, including, without limitation, risks and uncertainties associated with the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, the availability of sufficient resources to fund our various product candidate development programs and to meet our business objectives and operational requirements, the fact that the results of earlier studies and trials may not be predictive of future clinical trial results, the protection and market exclusivity provided by our intellectual property, risks related to the drug development and the regulatory approval process, including the timing and requirements of regulatory actions, and the impact of competitive products and technological changes. We disclaim any intent or obligation to update these forward-looking statements to reflect events or circumstances that exist after the date on which they were made. You should review additional disclosures we make in our filings with the Securities and Exchange Commission, including our Annual Report on Form 10-K and Quarterly Report on Form 10-Q. You may access these documents for no charge at http://www.sec.gov.
Corporate Contact:
Jim DeNike
Acer Therapeutics Inc.
+1-844-902-6100
[email protected]
Investor Relations Contact:
Nick Colangelo
Gilmartin Group
+1-339-225-1047
[email protected]