We are a pharmaceutical company focused on the acquisition, development, and commercialization of therapies for serious rare and life-threatening diseases with significant unmet medical needs. We identify and develop treatments where science can be applied in new ways for use in diseases with high unmet need.
In the U.S., OLPRUVA™ (sodium phenylbutyrate) is approved for the treatment of urea cycle disorders (UCDs) involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC), or argininosuccinic acid synthetase (AS). Acer is also advancing a pipeline of investigational product candidates for rare and life-threatening diseases, including: OLPRUVA™ (sodium phenylbutyrate) for treatment of various disorders, including Maple Syrup Urine Disease (MSUD); and EDSIVO™ (celiprolol) for treatment of vascular Ehlers-Danlos syndrome (vEDS) in patients with a confirmed type III collagen (COL3A1) mutation.
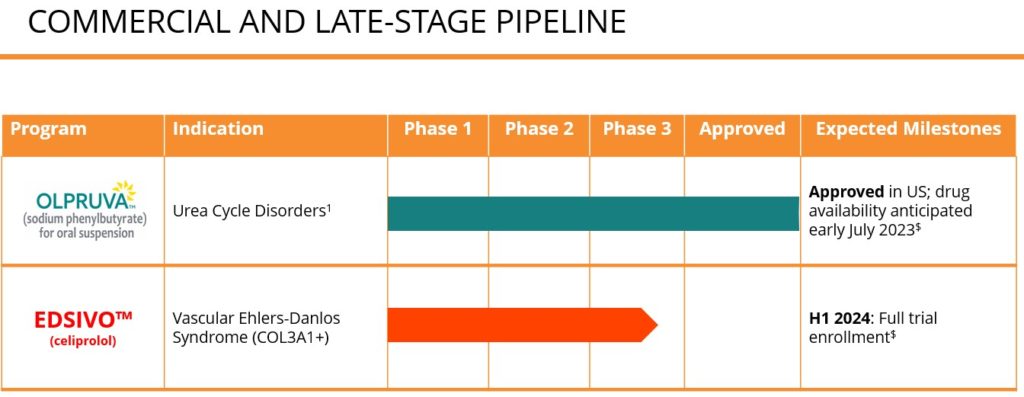
1 OLPRUVA™ (sodium phenylbutyrate) for oral suspension approved in the U.S. for the treatment of certain patients living with urea cycle disorders (UCDs) involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC), or argininosuccinic acid synthetase (AS)
$ Subject to additional capital
In the U.S., OLPRUVA™ (sodium phenylbutyrate) is indicated for the treatment of certain patients living with urea cycle disorders (UCDs) involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC), or argininosuccinic acid synthetase (AS). EDSIVO™ (celiprolol) is a product candidate under investigation and its safety and efficacy has not been established. There is no guarantee that this product will receive health authority approval or become commercially available for the uses being investigated.