In March 2021, we announced that Acer and Relief entered into a Collaboration and License Agreement (CLA) for worldwide development and commercialization of ACER-001. ACER-001 is a proprietary immediate release multi-particulate powder formulation of sodium phenylbutyrate (NaPB) with a taste-masked coating. The formulation consists of a core center, a layer of active drug, and a taste-masked coating designed to avoid the bitter taste in the mouth while quickly dissolving in the low pH of the stomach. ACER-001’s taste-masked formulation is aimed to improve the palatability of NaPB.
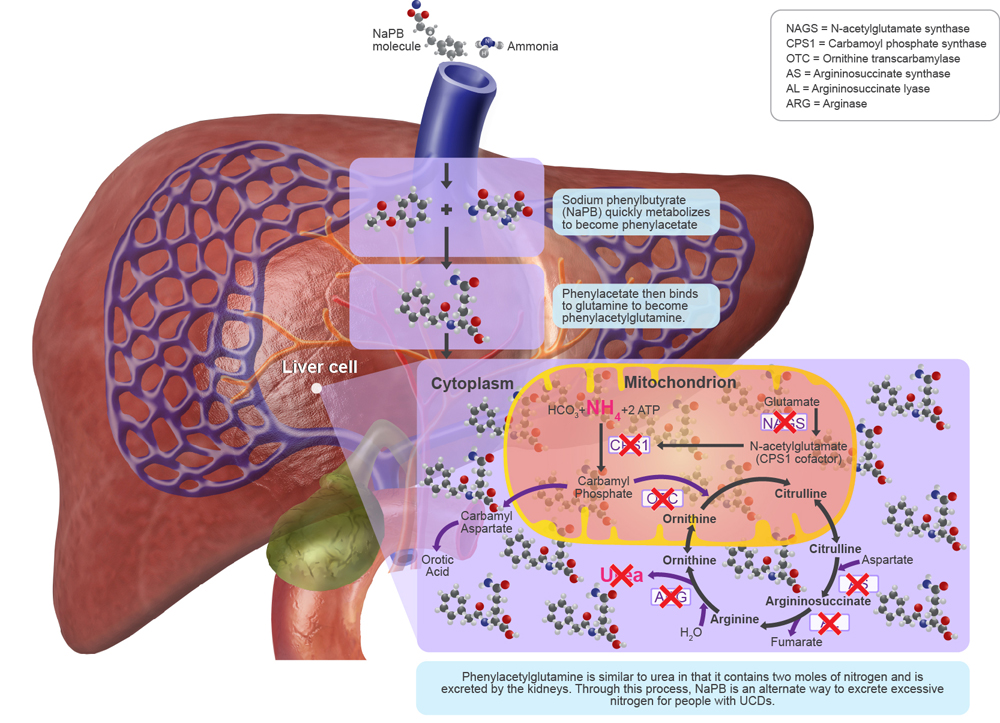
For the treatment of UCDs, NaPB is a prodrug that metabolizes quickly to become phenylacetate. Phenylacetate then binds to glutamine via acetylation to become phenylacetylglutamine. Phenylacetylglutamine is similar to urea in that it contains two moles of nitrogen and is excreted by the kidneys. Through this process, NaPB is an alternate way to excrete excessive nitrogen created by ineffective processing of ammonia during the urea cycle.1
ACER-001’s taste-masked formulation is designed to improve the palatability of NaPB. Acer has been granted orphan drug designation by the FDA for the MSUD indication. ACER-001 is under clinical investigation and its safety and efficacy have not been established. There is no guarantee that this product candidate will receive FDA approval or become commercially available for the uses being investigated.
ACER-001 Bridging Study Under Fasted Conditions
In February 2020, we reported the completion and final data from our clinical trial evaluating the bioavailability and bioequivalence of ACER-001 to BUPHENYL® (NaPB) both under fasted conditions. The trial was a single-center, single-blind, randomized, single-dose crossover study designed to show bioequivalence of ACER-001 compared to BUPHENYL® in 36 healthy adult subjects under fasted conditions. Data showed ACER-001 to have similar pharmacokinetic (PK) profiles for both phenylbutyrate (PBA) and phenylacetate (PAA) compared to BUPHENYL® under fasted conditions.
This trial also included an arm of ACER-001 administered under fed conditions. When the fed and fasted arms of the study were compared, it was shown that administration of ACER-001 in a fasted state achieved more than two times the maximum concentration (Cmax) of PBA compared to administration of the same dose of ACER-001 in a fed state. These results are consistent with previously published data by Nakano, et al3 that evaluated PK of NaPB in patients with progressive familial intrahepatic cholestasis, also demonstrating that administration of NaPB in a fasted state significantly increased PBA peak plasma concentration compared to administration of NaPB in a fed state. In addition, results from Osaka, et al4 also showed that “preprandial administration of NaPB may be preferable to maximize its therapeutic potency, allow a decrease in the clinically effective dose, and prevent BCAA depletion in UCD patients.”
Currently approved therapies for UCDs, including BUPHENYL®5 and RAVICTI®6, are required to be administered with food. BUPHENYL® is required to be administered in a fed state due to its aversive odor and taste, with side effects including nausea, vomiting and headaches, which can lead to discontinuation of treatment.7 Additionally, prescribing information states that the BUPHENYL® food effect is unknown. RAVICTI® PK and pharmacodynamic (PD) properties were determined to be indistinguishable in fed or fasted states.8 ACER-001 is uniquely formulated with its multi-particulate, taste-masked coating to allow for administration in a fasted state, while still allowing for rapid systemic release.
Based on the results from the food effect study within the ACER-001 BE trial, we commissioned Rosa & Co. LLC to create a PhysioPD® PK model to evaluate the potential food effect on exposure, tolerability and efficacy of ACER-001 in UCDs patients. Results from this in silico model suggested that administration of ACER-001 in a fasted state required approximately 30% less PBA to achieve comparable therapeutic benefit in a fed state. In addition, the model predicted that administration of ACER-001 in a fasted state compared to administration of BUPHENYL® or RAVICTI® (same amounts of PBA) in their required fed states would be expected to result in higher peak blood PBA, PAA and PAGN concentrations, predicting a 43% increase in urinary PAGN levels (a negative correlation between blood ammonia area under the curve and 24-hour urinary PAGN amount has been demonstrated).
ACER-001 Bridging Study Under Fed Conditions
In February 2021, we announced topline results from our bioequivalence trial in which ACER-001 showed similar relative bioavailability to BUPHENYL® under fed conditions. The single-center, single-blind, randomized, single-dose crossover trial evaluated BE of ACER-001 compared to BUPHENYL® when administered under fed conditions in 36 healthy adults. The topline data from this fed trial showed ACER-001 to have similar PK profiles for both PBA and PAA compared to BUPHENYL® under fed conditions.
ACER-001 Registration Plan
Following our Type B pre-NDA meeting with the FDA in the second quarter of 2021, we are planning an NDA submission in Q3 2021, provided we obtain agreement with FDA on our initial pediatric study plan. We are pursuing the planned submission under the Section 505(b)(2) regulatory pathway of ACER-001 for the treatment of patients with UCDs.
In parallel or after initial potential FDA approval for administration under fed conditions, and subject to additional capital, we also plan to evaluate the potential development of ACER-001 for administration under fasted (pre-meal) conditions, which will likely require additional nonclinical and clinical studies, in order to provide the necessary evidence of safety and efficacy of ACER-001 to be considered for FDA approval for administration under fasted (pre-meal) conditions.
More information on ACER-001 and our other programs can be found in our current corporate presentation. ACER-001 is an investigational drug in the U.S. and is not currently FDA approved for UCDs.
References
- Brusilow SW, Maestri NE. Urea cycle disorders: diagnosis, pathophysiology, and therapy. Adv Pediatr. 1996;43:127-170.
- Shchelochkov OA,et al. Barriers to drug adherence in the treatment of urea cycle disorders: Assessment of patient, caregiver and provider perspectives. Mol Genet Metab. 2016;8:43-47.
- Nakano S, et al. Effect of food on the pharmacokinetics and therapeutic efficacy of 4-phenylbutyrate in progressive familial intrahepatic cholestasis. Sci Rep 9, 17075 (2019).
- Osaka S, et al. A randomized trial to examine the impact of food on pharmacokinetics of 4-phenylbutyrate in healthy volunteers. Mol Genetics and Metab. Feb. 2021.
- https://www.hzndocs.com/BUPHENYL-Prescribing-Information.pdf
- https://www.hzndocs.com/RAVICTI-Prescribing-Information.PDF
- Pena-Qintana L, et al. Profile of sodium phenylbutyrate granules for the treatment of urea-cycle disorders: patient perspectives. Patient Preference and Adherence Volume 11:1489-1496, September 2017.
- United States Patent number US8642012B2.