In May 2020, we announced that we had entered into a research collaboration agreement with the National Center for Advancing Translational Sciences, or NCATS, one of the National Institutes of Health, or NIH, to develop emetine hydrochloride as a potential treatment of SARS-CoV-2 infection, a virus that causes COVID-19. ACER-2820 (emetine) is an active pharmaceutical ingredient of syrup of ipecac, given orally to induce emesis, and has also been formulated as an injectable to treat thousands of individuals with amebiasis. Several independent emetine in vitro studies have demonstrated nanomolar potency against both DNA and RNA-replicating viruses, including Zika virus, Ebola virus1, Rabies Lyssavirus, human cytomegalovirus, human immunodeficiency virus 1, influenza A virus, Rift Valley fever virus, echovirus 1, human metapneumovirus, and herpes simplex virus type 22. Clinically, emetine has been used to treat approximately 700 patients (including pediatrics) with viral hepatitis3 and varicella-zoster virus4. Additionally, emetine is a potent inhibitor of multiple genetically-distinct coronaviruses and demonstrated in vitro the strongest anti-coronavirus activity in one study that screened and identified approved compounds with broad-spectrum efficacy against the replication of four coronaviruses5 and specifically against SARS-CoV-2.6
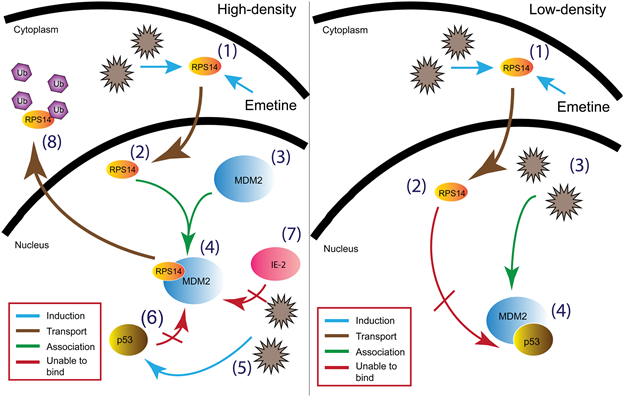
Figure: In high-density infected cells (A) emetine induces (1) nuclear translocation of RPS14 (2) followed by RPS14 binding to MDM2 (3 & 4) resulting in disruption of the interaction between MDM2-p53 (6) and MDM2- viral IE2 (5 & 7), and by RPS14 ubiquitination and degradation (8). In low-density infected cells (B) although emetine induces (1) nuclear translocation of RPS14 (2), it is unable to interact with MDM2 (4) which is already bound to p53 to facilitate virus replication (3). Source: Source: PLoS Pathogens 12(6):e1005717, June 2016
ACER-2820 Registration Plan
Further advancement of the ACER-2820 program for the treatment of certain viruses, including cytomegalovirus, zika, dengue, ebola, and COVID-19 is dependent on our ability to raise non-dilutive capital for this program. Additional information on the emetine program can be found in our current corporate presentation.
ACER-2820 is an investigational drug in the U.S. and is not currently FDA approved for any indication.
References
- Yang S, et al. Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: inhibiting viral replication and decreasing viral entry. Cell Discov (2018) 4:31. doi:10.1038/s41421-018-0034-1.
- Andersen, P.I., et al. Novel Antiviral Activities of Obatoclax, Emetine, Niclosamide, Brequinar, and Homoharringtonine. Viruses 2019, 11, 964.
- Del Puerto et al. Pren. méd. argent., 55: 818, 1968
- Annamalai et al. Emetine Hydrochloride in the Treatment of Herpes Zoster. 1968
- Shen L, et al. High-Throughput Screening and Identification of Potent Broad-Spectrum Inhibitors of Coronaviruses. J Virol. 2019 May 29;93(12).
- Choy et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020 Jun; 178: 104786