U.S. OLPRUVA™ launch progressing ahead of schedule with drug-in-channel now anticipated in mid-June 2023
NEWTON, MA – May 15, 2023 – Acer Therapeutics Inc. (Nasdaq: ACER), a pharmaceutical company focused on the acquisition, development and commercialization of therapies for serious, rare and life-threatening diseases with significant unmet medical needs, today reported financial results for the first quarter ended March 31, 2023 and provided a corporate update.
“The first quarter of 2023 was marked by considerable progress and a number of significant milestones in support of our commercial launch of OLPRUVA™, an innovative treatment option for patients with certain Urea Cycle Disorders (UCDs),” said Chris Schelling, CEO and Founder of Acer. “As a result, we are now ahead of our anticipated launch schedule with drug-in-channel expected in mid-June 2023. We have also made significant progress in the other key areas of our OLPRUVA™ commercialization strategy, including ongoing discussions with commercial and government insurance providers, physician outreach and awareness, and patient support and fulfillment. We look forward to continued progress in all of these areas and to delivering OLPRUVA™ to patients in mid-June 2023.”
Mr. Schelling added, “We have also started a broad outreach program to physicians who treat vascular Ehlers-Danlos Syndrome (vEDS) patients and have received overwhelming support for our ongoing Decentralized Study of Celiprolol on vEDS-related Event Reduction (DiSCOVER) Phase 3 EDSIVO™ (celiprolol) clinical trial. As a result, we anticipate enrollment in this trial to be completed by the end of this year. All of these activities are subject to additional capital.”
Q1 2023 and Recent Highlights
- OLPRUVA™ (sodium phenylbutyrate) for oral suspension
- In March 2023, Acer announced the presentation of data at the Annual Meeting of the Society for Inherited Metabolic Disorders (SIMD) from a survey of UCD healthcare providers sponsored by Acer that indicated taste and odor were the most important attributes when considering treatment options and treatment adherence
- Also at the SIMD Annual Meeting, Acer representatives met with 33 metabolic treatment providers — including nurse practitioners, registered dieticians, and physicians – from 24 metabolic treatment centers throughout the U.S. Of those metabolic treatment providers surveyed by Acer, 70% expressed a high interest in treating at least one of their patients with OLPRUVA™ in 2023
- In preparation for OLPRUVA™’s planned drug-in-channel in mid-June 2023, the Company is in discussions with the major pharmacy benefits managers (PBM) and group purchasing organizations (GPO) representing a substantial majority of covered lives
- Acer has also established a responsible and competitive pricing strategy designed to offer UCD patients a new treatment option at a significant discount to RAVICTI® while delivering predictable pricing that will not increase beyond the rate of inflation. Acer also plans to invest a portion of OLPRUVA™ revenue back into additional solutions aimed at improving outcomes for UCD patients
- Finally, Acer has established and staffed its patient support program, Navigator by Acer Therapeutics, that includes a suite of services designed to provide streamlined and efficient prescription management — including benefits verification, education, and home delivery — and personalized support for OLPRUVA™ patients
- EDSIVO™ (celiprolol)
- Acer continues to enroll patients in its ongoing, pivotal Phase 3 Decentralized Study of Celiprolol on vEDS-related Event Reduction (DiSCOVER) clinical trial of EDSIVO™ (celiprolol) in patients with COL3A1-positive vEDS. The double-blind portion of the DiSCOVER trial is designed to include an interim analysis conducted after 28 vEDS related events, which could occur as early as approximately 18 months after completion of full enrollment; and end if statistical significance is reached after 46 vEDS-related events, estimated to occur as early as approximately 40 months after completion of full enrollment
- ACER-801
- In March 2023, Acer announced topline results from its Phase 2a trial evaluating ACER-801 (osanetant) for the treatment of moderate to severe Vasomotor Symptoms (VMS) associated with menopause. The trial showed that ACER-801 was generally safe and well-tolerated but did not achieve statistically significant decrease in frequency or severity of hot flashes in postmenopausal women. As a result, Acer has paused the ACER-801 program until it has completed a thorough review of the full data set from the Phase 2a trial
- Corporate
- Ended Q1 2023 with $6.4 million in cash and cash equivalents. Acer believes its cash and cash equivalents available at March 31, 2023, together with $0.4 million from Acer’s ATM facility subsequent to March 31, 2023, will be sufficient to fund its anticipated operating and capital requirements into late in the second quarter of 2023
Anticipated Milestones (Subject to Available Capital)
- Mid-to-Late May 2023: Acer expects to publish OLPRUVA™’s list price, or wholesale acquisition cost (WAC), in mid-to-late May 2023
- June 2023: Acer anticipates OLPRUVA™ drug availability in mid-June 2023
- 2H 2023: Acer expects to begin attaining OLPRUVA™ commercial insurance coverage in 2H 2023
- Q3 2023: Acer expects to begin attaining insurance coverage for OLPRUVA™ Medicaid patients starting in Q3 2023
- Year End 2023: Acer anticipates enrollment completion by end of 2023 in its ongoing, pivotal Phase 3 DiSCOVER trial of EDSIVO™ in patients with COL3A1-positive vEDS
- Acer also intends to pursue additional opportunities for potential OLPRUVA™ label expansion, including the potential for additional dosage strengths to address patients with lower weights/body surface areas, and potential administration using a gastrostomy tube (G-tube)
Q1 2023 Financial Results
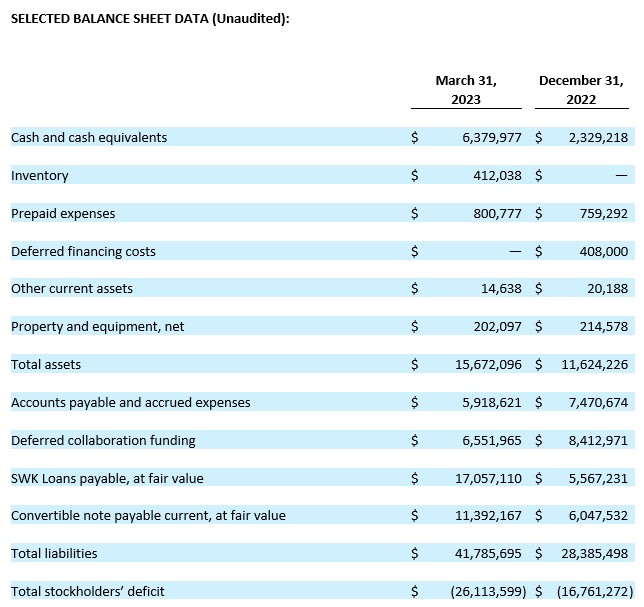
Cash Position. Cash and cash equivalents were $6.4 million as of March 31, 2023, compared to $2.3 million as of December 31, 2022. Acer believes its cash and cash equivalents available at March 31, 2023, together with $0.4 million from Acer’s ATM facility subsequent to March 31, 2023, will be sufficient to fund its anticipated operating and capital requirements into late in the second quarter of 2023.
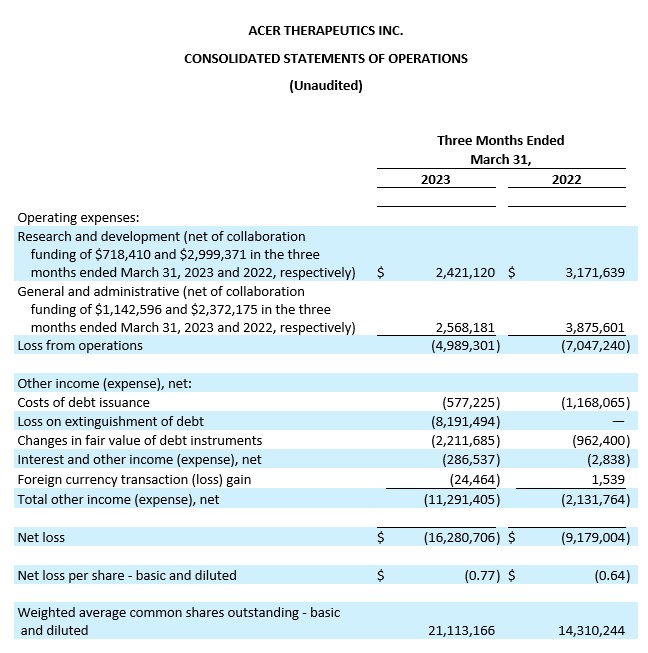
Research and Development Expenses. Research and development expenses were $2.4 million, net of collaboration funding of $0.7 million, for the three months ended March 31, 2023, as compared to $3.2 million, net of collaboration funding of $3.0 million, for the three months ended March 31, 2022. This decrease of $0.8 million was primarily due to decreases in expenses for contract manufacturing and contract research, employee-related expenses, and clinical studies related to ACER-801 and EDSIVOTM. Research and development expenses related to ACER-001 decreased in the three months ended March 31, 2023, resulting in a decrease in the recognition of the collaboration funding from the Collaboration Agreement with Relief. Research and development expenses for the three months ended March 31, 2023 were comprised of $1.0 million related to EDSIVOTM; $0.8 million related to ACER-001, offset by $0.7 million of collaboration funding; $0.8 million related to ACER-801; and $0.5 million related to other development activities.
General and Administrative Expenses. General and administrative expenses were $2.6 million, net of collaboration funding of $1.1 million, for the three months ended March 31, 2023, as compared to $3.9 million, net of collaboration funding of $2.4 million, for the three months ended March 31, 2022. This decrease of $1.3 million was primarily due to decreases in employee-related expenses, precommercial expenses, and consulting fees. General and administrative expenses related to ACER-001 decreased in the three months ended March 31, 2023, resulting in a decrease in the recognition of the collaboration funding from the Collaboration Agreement with Relief.
Net Loss. Net loss for the three months ended March 31, 2023 was $16.3 million, or $0.77 net loss per share (basic and diluted), compared to a net loss of $9.2 million, or $0.64 net loss per share (basic and diluted), for the three months ended March 31, 2022.
For additional information, please see Acer’s Quarterly Report on Form 10-Q filed today with the Securities and Exchange Commission (SEC).
About Acer Therapeutics
Acer is a pharmaceutical company focused on the acquisition, development and commercialization of therapies for serious rare and life-threatening diseases with significant unmet medical needs. In the U.S., OLPRUVA™ (sodium phenylbutyrate) is approved for the treatment of urea cycle disorders (UCDs) involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC), or argininosuccinic acid synthetase (AS). Acer is also advancing a pipeline of investigational product candidates for rare and life-threatening diseases, including: OLPRUVA™ (sodium phenylbutyrate) for treatment of various disorders; EDSIVO™ (celiprolol) for treatment of vascular Ehlers-Danlos syndrome (vEDS) in patients with a confirmed type III collagen (COL3A1) mutation; and ACER-801 (osanetant) for treatment of Vasomotor Symptoms (VMS), post-traumatic stress disorder (PTSD) and prostate cancer. For more information, visit www.www.acertx.com.
Acer Forward-Looking Statements
This press release contains “forward-looking statements” that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in this press release are forward-looking statements. Examples of such statements include, but are not limited to, statements about plans and strategy for the commercialization of OLPRUVA™ for oral suspension in the U.S. for the treatment of certain patients with certain UCDs, including launch schedule and timing of drug in channel, progress with respect to discussions with commercial and government insurance providers, physicians outreach and awareness, and patient support and fulfillment, statements with respect to our EDSIVO clinical trial for patients with vEDS, including enrollment and timing milestones related thereto, statements about our anticipated 2023 milestones, statements about our investment of OLPRUVA revenue, and statements about our capital requirements and sufficiency and duration of our current cash and cash equivalents. Our efforts to commercialize OLPRUVA™ for oral suspension in the U.S. for the treatment of certain patients with UCDs involving deficiencies of CPS, OTC, or AS are at an early stage, we currently do not have fully developed marketing, sales or distribution capabilities, and there is no guarantee that we will be successful in our commercialization efforts. Our pipeline products (including OLPRUVA™ for indications other than UCDs as well as EDSIVO™ and ACER-801) are under investigation and their safety and efficacy have not been established and there is no guarantee that any of our investigational products in development will receive health authority approval or become commercially available for the uses being investigated. We may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in the forward-looking statements and you should not place undue reliance on these forward-looking statements. Such statements are based on management’s current expectations and involve risks and uncertainties. Actual results and performance could differ materially from those projected in the forward-looking statements as a result of many factors, including, without limitation, the availability of financing to fund our commercialization efforts, our pipeline product development programs and our general corporate operations as well as risks related to drug development and the regulatory approval process, including the timing and requirements of regulatory actions. We disclaim any intent or obligation to update these forward-looking statements to reflect events or circumstances that exist after the date on which they were made. You should review additional disclosures we make in our filings with the Securities and Exchange Commission, including our Annual Report on Form 10-K and Quarterly Reports on Form 10-Q. You may access these documents for no charge at http://www.sec.gov.
Corporate and IR Contact
Jim DeNike
Acer Therapeutics Inc.
[email protected]
+1-844-902-6100
Nick Colangelo
Gilmartin Group
[email protected]
+1-332-895-3226