NEWTON, MA –August 10, 2021 – Acer Therapeutics Inc. (Nasdaq: ACER), a pharmaceutical company focused on the acquisition, development and commercialization of therapies for serious rare and life-threatening diseases with significant unmet medical needs, today reported financial results for the second quarter ended June 30, 2021 and provided an update on the Company’s recent corporate developments.
“This quarter marks significant, meaningful progress across our entire pipeline, including the very recent submission of our NDA for ACER-001 for the treatment of UCDs,” said Chris Schelling, CEO and Founder of Acer. “As we anticipate working closely with the FDA on the review process for ACER-001, we continue to make significant progress across the rest of our pipeline, including preparation of two IND submissions targeted for Q4 of this year. These INDs are expected to support initiating a dose-ranging Phase 2a study for ACER-801 (osanetant) for the treatment of vasomotor symptoms, and a pivotal Phase 3 study for EDSIVO™ for the treatment of COL3A1+ vEDS. On the corporate front, we have enhanced our management team with four important senior-level hires across their respective disciplines, including program and alliance management, marketing, and clinical development. I warmly welcome our new team members, who bring deep industry knowledge and tremendous experience, and look forward to their contributions as we position ourselves for growth.”
Q2 2021 and Recent Highlights
- ACER-001 (sodium phenylbutyrate)
- Following completion of a Q2 2021 pre-NDA meeting with the U.S. Food and Drug Administration (FDA), submitted a New Drug Application (NDA) in August 2021 for ACER-001 for the treatment of Urea Cycle Disorders (UCDs)
- Reached agreement with FDA on the Company’s initial Pediatric Study Plan (iPSP) which outlines an approach that addresses the needs of pediatric patients with UCDs
- ACER-801 (osanetant)
- Continued to advance toward an Investigational New Drug Application (IND) submission for the treatment of vasomotor symptoms (VMS)
- EDSIVO™ (celiprolol)
- Following completion of a Type B meeting with FDA, in June 2021 Acer announced plans to conduct a pivotal Phase 3, randomized, double-blind, placebo-controlled, decentralized clinical trial for EDSIVO™ for patients with COL3A1+ vascular Ehlers-Danlos Syndrome (vEDS), subject to available capital
- The DiSCOVER (Decentralized Study of Celiprolol on vEDS-related Event Reduction) trial is estimated to take approximately 3.5 years to complete, once fully enrolled
- FDA indicated agreement with Acer’s plans to submit a proposed protocol under the IND Special Protocol Assessment (SPA) process with possible breakthrough designation
- Launched www.discoverceliprolol.com as an outreach tool for interested parties
- Following completion of a Type B meeting with FDA, in June 2021 Acer announced plans to conduct a pivotal Phase 3, randomized, double-blind, placebo-controlled, decentralized clinical trial for EDSIVO™ for patients with COL3A1+ vascular Ehlers-Danlos Syndrome (vEDS), subject to available capital
- Corporate
- Hired Tanya Hayden, Vice President of Program and Strategic Alliance Management, in June 2021. During her nearly 20-year tenure at Lonza (formerly Bend Research/Capsugel), Ms. Hayden was responsible for oral drug delivery technologies, clinical and commercial contract manufacturing, operational excellence and client-focused project management
- Hired John Hilton, Vice President of Marketing and Strategic Insights, in July 2021. Mr. Hilton brings over 20 years of biopharmaceutical industry experience including marketing, sales, KOL development, pipeline marketing, and access strategy functions, as well as oversight of launch planning for ten new products in the last ten years
- Hired Terrie Kellmeyer, Ph.D., Vice President of Clinical Development, in August 2021. Dr. Kellmeyer brings to Acer over 23 years of experience in the pharmaceutical industry spanning the areas of Clinical Development, Regulatory Affairs, Medical Writing and Medical Affairs
- Hired Jeffrey Edwards, Ph.D., Vice President of Clinical Sciences, in August 2021. Dr. Edwards brings to Acer over 16 years of pharmaceutical experience in clinical and preclinical studies and support for regulatory submissions
- Ended Q2 2021 with $22.1 million in cash and cash equivalents. Acer believes its cash and cash equivalents available as of June 30, 2021, plus a $10.0 million Second Development Payment conditioned upon FDA accepting an NDA for ACER-001 in a UCD for filing and review per the Collaboration Agreement with Relief Therapeutics, will be sufficient to fund its currently anticipated operating and capital requirements into mid-2022, excluding support for the planned ACER-801 and EDSIVO™ clinical trials
Anticipated Milestones
- ACER-001 (sodium phenylbutyrate)
- Q4 2021: Based on standard FDA review timelines, Acer expects to receive notification from FDA on the potential acceptance of the NDA for filing within 60 days of submission and subsequent substantive review
- ACER-001 is an investigational product candidate which has not been approved by FDA. There is no guarantee that this product candidate will be accepted for substantive review, or if accepted, receive regulatory authority approval in any territory, or become commercially available for the indications under investigation
- ACER-801 (osanetant)
- Q4 2021: IND submission for the treatment of VMS is anticipated in Q4 2021
- Q1 2022: Phase 2a clinical trial initiation is expected in Q1 2022 designed to evaluate ACER-801’s PK, efficacy and safety in postmenopausal women and to identify the optimal dosing strategy for subsequent efficacy studies in certain cancer patients with induced vasomotor symptoms (iVMS), dependent upon successful IND submission and clearance, and subject to additional capital
- EDSIVO™ (celiprolol)
- Q4 2021: Submission of the proposed EDSIVO™ pivotal trial protocol and IND, request for breakthrough therapy designation, coordination with FDA on SPA agreement, and beginning to identify COL3A1+ vEDS patients for potential trial participation are anticipated in Q4 2021
- Q1 2022: Initiation of the EDSIVO™ DiSCOVER pivotal clinical trial is expected in Q1 2022 subject to successful IND submission and clearance, FDA SPA agreement, and obtaining additional capital required to conduct and complete the trial
- ACER-2820 (emetine)
- Ongoing: With most of the emetine IND-enabling work complete, further advancement of the emetine program for treatment of certain infectious diseases, including COVID-19, is dependent on Acer’s ability to raise non-dilutive capital
Q2 2021 Financial Results
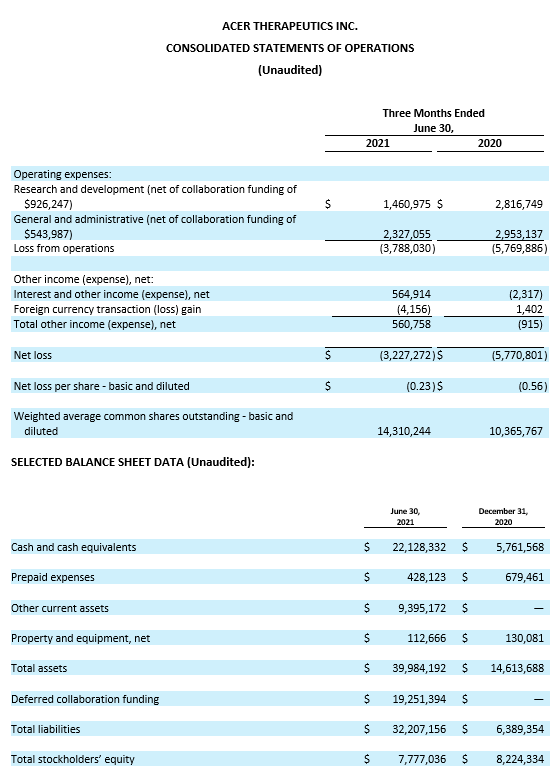
Cash position. Cash and cash equivalents were $22.1 million as of June 30, 2021, compared to $5.8 million as of December 31, 2020. Acer believes its cash and cash equivalents available as of June 30, 2021, plus a $10.0 million Second Development Payment conditioned upon FDA accepting an NDA for ACER-001 in a UCD for filing and review per the Collaboration Agreement with Relief Therapeutics, will be sufficient to fund its currently anticipated operating and capital requirements into mid-2022, excluding support for planned ACER-801 and EDSIVO™ clinical trials.
Research and Development Expenses. Research and development expenses were $1.5 million, net of collaboration funding of $0.9 million for the three months ended June 30, 2021, as compared to $2.8 million for the three months ended June 30, 2020. This decrease of $1.3 million was primarily due to the recognition of the $0.9 million of collaboration funding from the Collaboration Agreement with Relief, as well as a decrease in expenses for contract research and contract manufacturing, partially offset by an increase in consulting and professional fees. Research and development expenses for the three months ended June 30, 2021 were comprised of $1.1 million related to ACER-001, offset by $0.9 million of collaboration funding; $0.5 million related to emetine; $0.3 million related to ACER-801; $0.3 million related to EDSIVOTM; and $0.2 million related to other development activities.
General and Administrative Expenses. General and administrative expenses were $2.3 million, net of collaboration funding of $0.5 million for the three months ended June 30, 2021, as compared to $3.0 million for the three months ended June 30, 2020. This decrease of $0.7 million was primarily due to the recognition of the $0.5 million of collaboration funding from the Collaboration Agreement with Relief, as well as a reduction in one-time costs related to the Lincoln Park agreement recognized in 2020, partially offset by an increase in employee-related expenses.
Net Loss. Net loss for the three months ended June 30, 2021 was $3.2 million, or $0.23 net loss per share (basic and diluted), compared to a net loss of $5.8 million, or $0.56 net loss per share (basic and diluted), for the three months ended June 30, 2020.
For additional information, please see Acer’s Quarterly Report on Form 10-Q filed today with the SEC.
About Acer Therapeutics Inc.
Acer is a pharmaceutical company focused on the acquisition, development and commercialization of therapies for serious rare and life-threatening diseases with significant unmet medical needs. Acer’s pipeline includes four programs: ACER-001 (sodium phenylbutyrate) for treatment of various inborn errors of metabolism, including urea cycle disorders (UCDs) and Maple Syrup Urine Disease (MSUD); ACER-801 (osanetant) for treatment of induced vasomotor symptoms (iVMS); EDSIVO™ (celiprolol) for treatment of vascular Ehlers-Danlos syndrome (vEDS) in patients with a confirmed type III collagen (COL3A1) mutation; and ACER-2820 (emetine), a host-directed therapy against a variety of infectious diseases, including COVID-19. Each of Acer’s product candidates is believed to present a comparatively de-risked profile, having one or more of a favorable safety profile, clinical proof-of-concept data, mechanistic differentiation and/or accelerated paths for development through specific programs and procedures established by the FDA. In March 2021, Acer entered into a Collaboration and License Agreement with Relief Therapeutics for development and commercialization of ACER-001. For more information, visit www.acertx.com.
Forward-Looking Statements
This press release contains “forward-looking statements” that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in this press release regarding strategy, future operations, timelines, future financial position, future revenues, projected expenses, regulatory submissions, actions or approvals, cash position, liquidity, prospects, plans and objectives of management are forward-looking statements. Examples of such statements include, but are not limited to, statements relating to the potential for our product candidates to safely and effectively treat diseases and to be approved for marketing; the commercial or market opportunity of any of our product candidates in any target indication and any territory; our ability to secure the additional capital necessary to fund our various product candidate development programs; the adequacy of our capital to support our future operations and our ability to successfully fund, initiate and complete clinical trials and regulatory submissions; the ability to protect our intellectual property rights; our strategy and business focus; and the development, expected timeline and commercial potential of any of our product candidates. We may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in the forward-looking statements and you should not place undue reliance on these forward-looking statements. Such statements are based on management’s current expectations and involve risks and uncertainties. Actual results and performance could differ materially from those projected in the forward-looking statements as a result of many factors, including, without limitation, risks and uncertainties associated with the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, the availability of sufficient resources to fund our various product candidate development programs and to meet our business objectives and operational requirements, the fact that the results of earlier studies and trials may not be predictive of future clinical trial results, the protection and market exclusivity provided by our intellectual property, risks related to the drug development and the regulatory approval process, including the timing and requirements of regulatory actions, and the impact of competitive products and technological changes. We disclaim any intent or obligation to update these forward-looking statements to reflect events or circumstances that exist after the date on which they were made. You should review additional disclosures we make in our filings with the Securities and Exchange Commission, including our Quarterly Reports on Form 10-Q and our Annual Report on Form 10-K. You may access these documents for no charge at http://www.sec.gov.
Investor Contact:
Hans Vitzthum
LifeSci Advisors
Ph: 617-430-7578
hans@lifesciadvisors.com
Jim DeNike
Acer Therapeutics Inc.
Ph: 844-902-6100
jdenike@acertx.com