NEWTON, MA – Nov. 13, 2019 – Acer Therapeutics Inc. (Nasdaq: ACER), a pharmaceutical company focused on the acquisition, development, and commercialization of therapies for serious rare and life-threatening diseases with significant unmet medical needs, today reported financial results for the third quarter ended September 30, 2019 and provided an update on the Company’s recent corporate developments.
“We have been very active as we continue to progress the development of our pipeline products, including continued work in support of potential EDSIVO™ approval, and the ongoing development of ACER-001,” said Chris Schelling, CEO and Founder of Acer. “We have completed our EDSIVO™ Type A meeting following receipt of the Complete Response Letter (CRL) and are evaluating plans to appeal the decision. At the same time, we are well underway with our ACER-001 pivotal bioequivalence trial for urea cycle disorders (UCDs), and we are advancing osanetant toward clinical development for induced Vasomotor Symptoms (iVMS), both as previously announced.”
Third Quarter 2019 and Recent Events
- EDSIVO™
- Conducted a Type A meeting in October 2019 with the U.S. Food and Drug Administration (FDA) regarding the EDSIVO™CRL and engaged with industry experts to determine an optimal path forward
- Presented data at the Academy of Managed Care Pharmacy (AMCP) Nexus 2019 conference from a pilot study designed to estimate the healthcare costs associated with clinical events in patients with vEDS in the United States. The poster can be found here: https://www.acertx.com/edsivo-publications-and-presentations/
- ACER-001
- Fully enrolled Part A of a two-part pivotal bioavailability and bioequivalence (BE) trial to bridge ACER-001 to BUPHENYL® for treatment of UCDs. Part A is a single-center, single-blind, randomized, single-dose crossover trial designed to evaluate the relative bioavailability of three different oral suspension formulations of ACER-001 compared to BUPHENYL® in 20 healthy adult subjects
- Initiated a taste assessment trial of three different formulations of ACER-001 (multi-particulate powder) assessed relative to BUPHENYL® (powder) using certified taste-testers
- Received Type C
written comments from the FDA in October 2019:
- Proceeding with clinical trials as planned
- The FDA stated that 12 months of long-term and 6 months of accelerated stability data would be required for submission of a 505(b)(2) New Drug Application (NDA)
- Ended the third quarter with $16.1 million in cash and cash equivalents, which the Company believes will be sufficient to fund its current operating and capital requirements through the end of 2020
Upcoming Milestones
- EDSIVO™
- Evaluating an appeal via Formal Dispute Resolution Request (FDRR) to the Office of New Drugs (OND) with potential submission by the end of 2019
- ACER-001
- Two-part pivotal BE trial:
- Results expected for Part A in Q4 2019
- Anticipate enrolling the first subjects in Part B in Q4 2019. Part B is a single-center, open-label, randomized, single-dose crossover trial to demonstrate bioequivalence of the optimal formulation of ACER-001 (chosen from Part A) compared to BUPHENYL® in 36 healthy adult subjects. Trial completion expected in Q1 2020
- Taste assessment trials:
- Results expected in Q4 2019 for taste assessment trial evaluating three different formulations of ACER-001
- Anticipate enrolling the first subjects in 1H 2020 for the taste study comparing the optimal ACER-001 formulation with BUPHENYL®. The design and size of the trial will be determined following further discussions with the FDA
- Submit NDA for UCDs in Q1 2021, subject to additional capital, assuming successful outcome in BE trial and 12-month long-term stability data
- Two-part pivotal BE trial:
- Osanetant
- Submit osanetant Investigational New Drug Application (IND) in Q2 2020
- Aim to initiate Phase 1/2 trial in 2H 2020, subject to additional capital, evaluating osanetant in patients with medically and/or surgically induced VMS in which Hormone Replacement Therapy (HRT) is contraindicated
Financial Results for the Third Quarter 2019
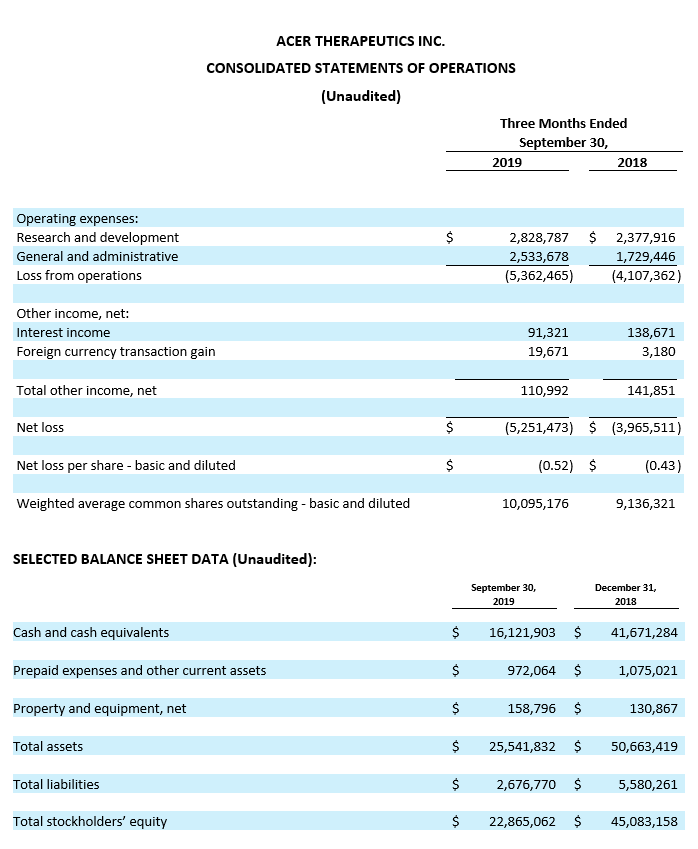
Cash position. Cash and cash equivalents were $16.1 million as of September 30, 2019, compared to $41.7 million as of December 31, 2018. The Company believes its cash position will be sufficient to fund its current operating and capital requirements through the end of 2020.
Research and Development Expenses. Research and development expenses were $2.8 million during the three months ended September 30, 2019, compared to $2.4 million during the three months ended September 30, 2018. This increase of $0.4 million was primarily due to increases in expenses related to manufacturing services, partially offset by decreases in employee-related expenses and in expenses related to research services and consulting services. Research and development expenses for the three months ended September 30, 2019 were primarily comprised of approximately $0.8 million related to EDSIVO™ and approximately $1.6 million related to ACER-001.
General and Administrative Expenses. General and administrative expenses were $2.5 million for the three months ended September 30, 2019 compared to $1.7 million for the three months ended September 30, 2018. The increase of $0.8 million was primarily due to increases in employee-related expenses.
Net Loss. Net loss for the three months ended September 30, 2019 was $5.3 million, or $0.52 net loss per share (basic and diluted), compared to a net loss of $4.0 million, or $0.43 net loss per share (basic and diluted), for the three months ended September 30, 2018. The increase in loss per share (basic and diluted) was driven largely by increases in expenses related to manufacturing services, partially offset by an increase in the number of shares outstanding at September 30, 2019 as compared to September 30, 2018.
For additional information, please see Acer’s Quarterly Report on Form 10-Q filed today with the SEC.
About Acer Therapeutics Inc.
Acer is a pharmaceutical company focused on the acquisition, development, and commercialization of therapies for serious rare and life-threatening diseases with significant unmet medical needs. Acer’s pipeline includes three clinical-stage pharmaceutical product candidates: EDSIVO™ (celiprolol), for the treatment of vascular Ehlers-Danlos syndrome (vEDS) in patients with a confirmed type III collagen (COL3A1) mutation; ACER-001 (a fully taste-masked, immediate-release formulation of sodium phenylbutyrate), for the treatment of various inborn errors of metabolism, including urea cycle disorders (UCDs) and Maple Syrup Urine Disease (MSUD); and osanetant, for the treatment of induced Vasomotor Symptoms (iVMS) where Hormone Replacement Therapy (HRT) is likely contraindicated. Each of Acer’s product candidates is believed to present a comparatively de-risked profile, having one or more of a favorable safety profile, clinical proof-of-concept data, mechanistic differentiation and/or accelerated paths for development through specific programs and procedures established by the FDA.
Forward-Looking Statements
This press release contains “forward-looking statements” that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in this press release regarding strategy, future operations, timelines, future financial position, future revenues, projected expenses, regulatory submissions, actions or approvals, cash position, liquidity, prospects, plans and objectives of management are forward-looking statements. Examples of such statements include, but are not limited to, statements relating to expectations regarding our capital resources; the potential for EDSIVO™ (celiprolol), ACER-001 and osanetant to safely and effectively treat diseases and to be approved for marketing; the commercial or market opportunity of any of our product candidates in any target indication and any territory; the adequacy of our capital to support our future operations and our ability to successfully initiate and complete clinical trials and regulatory submissions; our progress toward possible approval for EDSIVO™ in light of the Complete Response Letter we received earlier this year; the ability to protect our intellectual property rights; our strategy and business focus; and the development, expected timeline and commercial potential of any of our product candidates. We may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in the forward-looking statements and you should not place undue reliance on these forward-looking statements. Such statements are based on management’s current expectations and involve risks and uncertainties. Actual results and performance could differ materially from those projected in the forward-looking statements as a result of many factors, including, without limitation, risks and uncertainties associated with the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, our ability to reduce our operating expenses and conserve cash on a net basis as a result of our prior or any future corporate restructuring initiative, the availability of sufficient resources to meet our business objectives and operational requirements, the fact that the results of earlier studies and trials may not be predictive of future clinical trial results, the protection and market exclusivity provided by our intellectual property, the substantial costs and diversion of management’s attention and resources which could result from pending securities litigation, risks related to the drug development and the regulatory approval process, including the timing of regulatory actions, and the impact of competitive products and technological changes. We disclaim any intent or obligation to update these forward-looking statements to reflect events or circumstances that exist after the date on which they were made. You should review additional disclosures we make in our filings with the Securities and Exchange Commission, including our Quarterly Reports on Form 10-Q and our Annual Report on Form 10-K. You may access these documents for no charge at http://www.sec.gov.
Investor Contact:
Hans Vitzthum
LifeSci Advisors
Ph: 617-430-7578
Jim DeNike
Acer Therapeutics Inc.
Ph: 844-902-6100